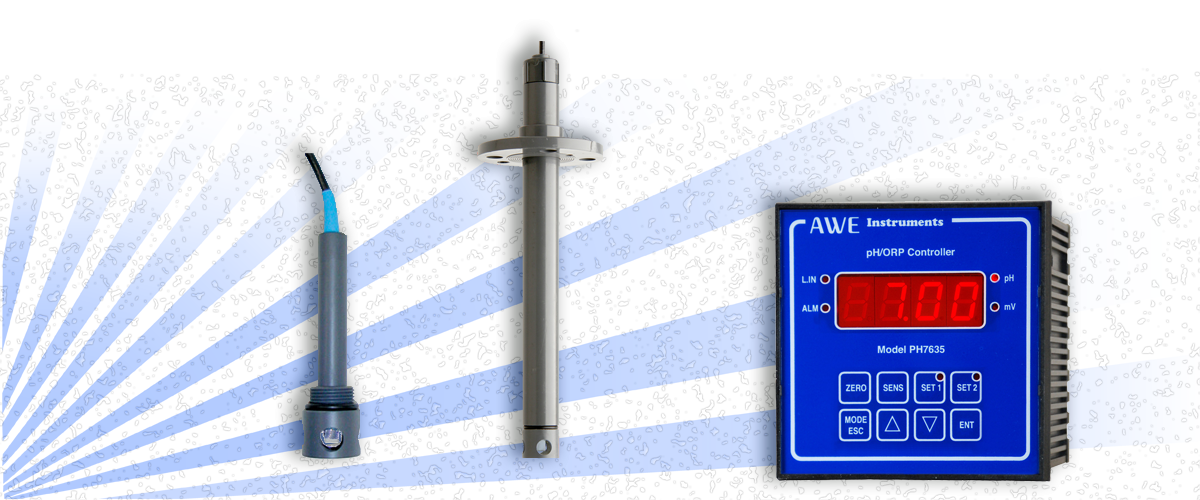
pH Probe for measuring pH in Hydrofluoric Acid (HF Acid) applications
For a long time a number of our customers have applications where a normal process pH electrode or pH probe has a very short service life.
This is normally because the chemical present is able to attack the glass in pH sensor. This is particularly true of one customer whose process was etching glass for production of shower enclosures!
If fluoride is present, this doesn't necessarily mean that the glass in pH electrode will be attacked, as Fluoride Ions alone (F-) do not attack glass. The Hydrofluoric Acid will attack glass, and is normally very aggressive. This is generally only present in the solution when the pH of solution being measured is less than 7pH.
If the application has a pH of 7 or lower then we would suggest using a pH probe for measuring pH in Hydrofluoric Acid, or an Antimony pH electrode.
Hydrofluoric Acid is able to etch glass (our shower enclosure customer was testament to this) which is where the problem for using glass pH electrodes arrises. The preferred alternative is to use a pH sensor which has an Antimony process electrode commonly referred to as an Antimony pH sensor.
In pH probes designed for measuring pH in Hydrofluoric Acid, the antimony reacts with an aqueous solution to form an oxide which releases hydrogen ions - as the hydrogen ions are active in the reaction, the pH changes can be calculated using specialist software in our pH controllers.
Considerations for using an Anitmony pH electrode
- When using a pH probe for measuring pH in Hydrofluoric Acid a number of considerations have to be made - firstly the range is restricted from 1 - 9pH.
- Secondly oxidising or reducing agents forming part of a redox reaction will have affect the measured result and should be avoided.
- The calibration of a pH probe which is designed for measuring pH in Hydrofluoric Acid is a slightly different process than that of a standard industrial pH probe. The antimony will oxidise in the solution resulting in a sluggish response to pH change values or no response at all if left unattended. During the periodic calibration simply remove the surface coating using a fine abrasive before carrying out the pH buffering process. The antimony is toxic so ensure suitable PPE is worn.
- Antimony does react with a number of ions which result in it becoming unusable. These include but are not limited to Copper, Tin and Lead. Simply submersing the antimony where these ions are present will stop the probe from working within a matter of minutes. In addition oxalates, tartrates and citrates can result in poor calibration - so we do strongly recommend using a certified pH buffer solution much like our range of AWE Instruments buffers.